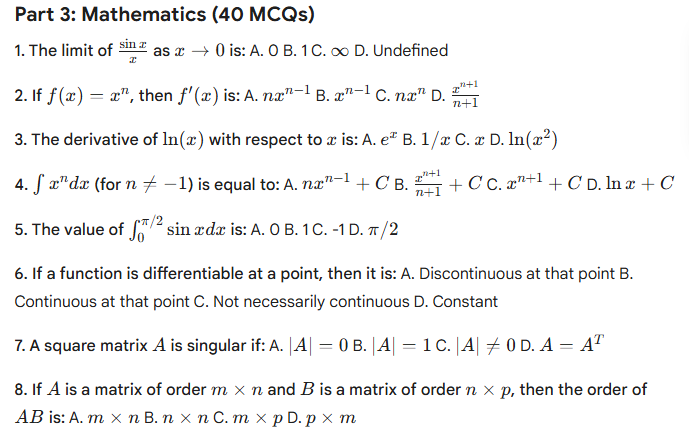
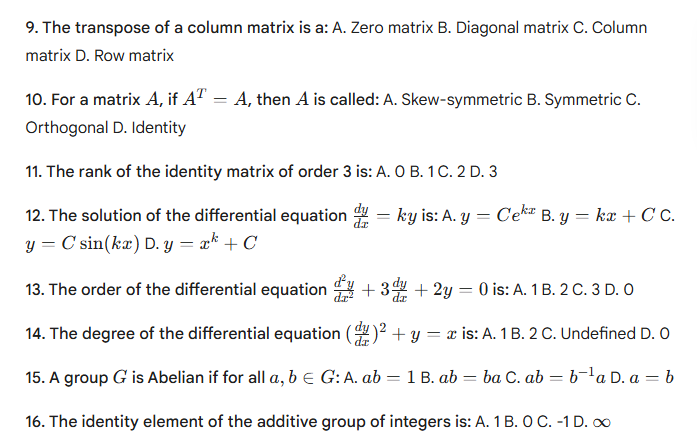
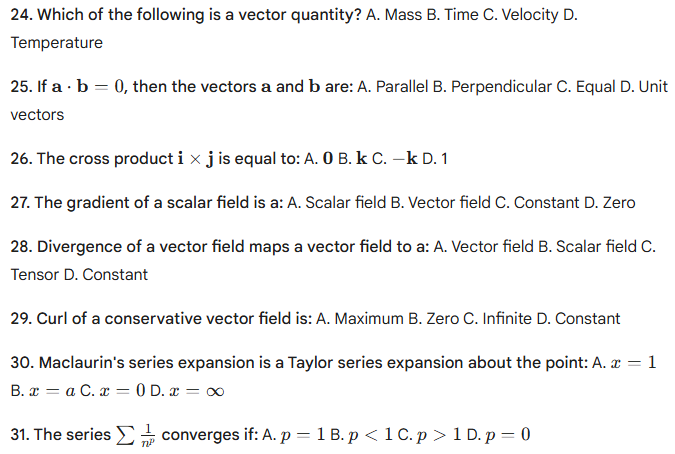
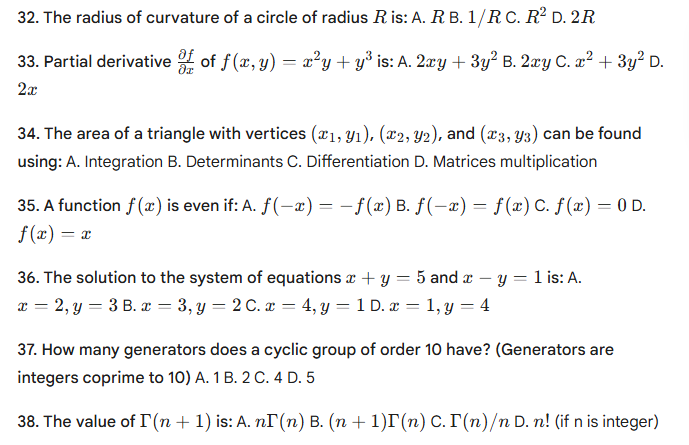HP TGT Non medical commission Mock Test







Part 2: Chemistry (40 MCQs)
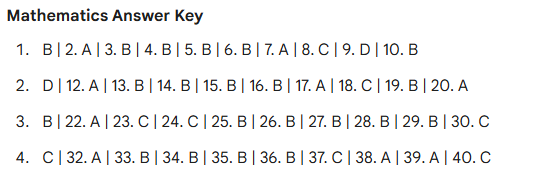
1. The shape of an $s$-orbital is: A. Dumbbell B. Double Dumbbell C. Spherical D. Complex
2. Which quantum number describes the orientation of the orbital in space? A. Principal quantum number ($n$) B. Azimuthal quantum number ($l$) C. Magnetic quantum number ($m$) D. Spin quantum number ($s$)
3. The maximum number of electrons that can be accommodated in a shell with principal quantum number $n$ is: A. $n$ B. $2n$ C. $n^2$ D. $2n^2$
4. According to VSEPR theory, the shape of the methane ($CH_4$) molecule is: A. Linear B. Trigonal planar C. Tetrahedral D. Octahedral
5. Which type of bond is formed by the sharing of electrons? A. Ionic bond B. Covalent bond C. Metallic bond D. Hydrogen bond
6. The bond order of the $O_2$ molecule is: A. 1 B. 1.5 C. 2 D. 2.5
7. Which of the following is an extensive property? A. Temperature B. Density C. Volume D. Pressure
8. For a spontaneous process, the change in Gibbs free energy ($\Delta G$) must be: A. Positive B. Negative C. Zero D. Infinite
9. The pH of a neutral solution at 25°C is: A. 0 B. 1 C. 7 D. 14
10. A buffer solution resists change in: A. Volume B. pH C. Temperature D. Concentration
11. The oxidation state of Cr in $K_2Cr_2O_7$ is: A. +3 B. +5 C. +6 D. +7
12. In a galvanic cell, oxidation takes place at the: A. Anode B. Cathode C. Salt bridge D. Electrolyte
13. The rate of a chemical reaction generally increases with: A. Decrease in temperature B. Increase in concentration of reactants C. Increase in concentration of products D. None of the above
14. A catalyst increases the rate of reaction by: A. Increasing the activation energy B. Decreasing the activation energy C. Increasing the enthalpy change D. Decreasing the enthalpy change
15. Which order of reaction has a half-life independent of initial concentration? A. Zero order B. First order C. Second order D. Third order
16. The phenomenon of scattering of light by colloidal particles is called: A. Tyndall effect B. Brownian movement C. Electrophoresis D. Dialysis
17. Alkanes have the general formula: A. $C_nH_{2n}$ B. $C_nH_{2n-2}$ C. $C_nH_{2n+2}$ D. $C_nH_{2n+1}$
18. Benzene is an example of: A. Alicyclic compound B. Aromatic compound C. Heterocyclic compound D. Aliphatic compound
19. The hybridization of carbon atoms in ethene ($C_2H_4$) is: A. $sp$ B. $sp^2$ C. $sp^3$ D. $sp^3d$
20. Lucas reagent is used to distinguish between: A. Aldehydes and Ketones B. Primary, Secondary, and Tertiary Alcohols C. Amines and Amides D. Acids and Esters
21. Oxidation of primary alcohols yields: A. Ketones B. Aldehydes C. Ethers D. Alkenes
22. Which acid is present in vinegar? A. Formic acid B. Acetic acid C. Citric acid D. Tartaric acid
23. Aniline on reaction with nitrous acid ($NaNO_2 + HCl$) at 0-5°C gives: A. Phenol B. Nitrobenzene C. Benzene Diazonium Chloride D. Chlorobenzene
24. Which of the following is a carbohydrate? A. Glycine B. Glucose C. Glycerol D. Glycol
25. Proteins are polymers of: A. Fatty acids B. Amino acids C. Glucose D. Nucleotides
26. In the periodic table, atomic size generally: A. Increases across a period B. Decreases down a group C. Decreases across a period and increases down a group D. Increases across a period and decreases down a group
27. The most electronegative element is: A. Oxygen B. Chlorine C. Nitrogen D. Fluorine
28. Transition elements are found in which block of the periodic table? A. s-block B. p-block C. d-block D. f-block
29. The coordination number of the central metal ion in $[Fe(CN)_6]^{3-}$ is: A. 2 B. 4 C. 6 D. 8
30. EDTA is a: A. Monodentate ligand B. Bidentate ligand C. Hexadentate ligand D. Tridentate ligand
31. Brass is an alloy of: A. Copper and Zinc B. Copper and Tin C. Copper and Nickel D. Iron and Carbon
32. Hardness of water is caused by soluble salts of: A. Sodium and Potassium B. Calcium and Magnesium C. Silver and Gold D. Lithium and Ammonium
33. The gas responsible for the Greenhouse effect is: A. $N_2$ B. $O_2$ C. $CO_2$ D. $Ar$
34. Ozone layer depletion is mainly caused by: A. $CO_2$ B. CFCs (Chlorofluorocarbons) C. $SO_2$ D. $CH_4$
35. Isomers having the same molecular formula but different structural formulas are called: A. Stereoisomers B. Structural isomers C. Enantiomers D. Conformers
36. A chiral carbon is bonded to: A. Two identical groups B. Three identical groups C. Four different groups D. Four identical groups
37. Which of the following acts as a Lewis acid? A. $NH_3$ B. $H_2O$ C. $BF_3$ D. $CH_4$
38. The unit of the rate constant for a zero-order reaction is: A. $s^{-1}$ B. $mol \cdot L^{-1} \cdot s^{-1}$ C. $L \cdot mol^{-1} \cdot s^{-1}$ D. Dimensionless
39. Phenol is less acidic than: A. Ethanol B. Methanol C. Carbonic acid D. Acetylene
40. Tollen’s reagent is used to detect: A. Ketones B. Aldehydes C. Alcohols D. Amines